Transition Element
Transition Elements also called transition metals, are elements whose d-orbitals are only partly filled. IUPAC says that transition elements are either an element whose d-subshell is only partially filled with electrons or an element that can form stable cations even though their d-orbital is only partially filled.
Some basic knowledge about Transition Element
- In general, transition elements are in the d-block of the modern periodic table. This block is made up of groups 3 to 12.
- d and f-block elements are called outer transition elements and inner transition elements respectively.
- There are four series of d-block elements i.e., 3d, 4d, 5d, and 6d and they are lying in periods 4, 5, 6, and 7 respectively.
- f-block elements are 4f and 5f and they are lying in periods 6 and 7.
- The elements of the 3d series are Sc21 to Zn30. 4d series are as Y39 to Cd48 . 5d series has La57, Hf72 to Hg80. The 6d series in the 7th period has only seven elements from 104 to 110. They are all called transition elements because their properties are in between s and p-block elements.
- The electronic distribution of the Cr and Cu families has one electron in their s- orbitals and 5 and 10 electrons in their d-orbitals respectively. The elements of II- B i.e., Zn, Cd, and Hg are non-typical transition elements. Similarly, the elements of III-B i.e., Sc, Y, and La are also non-typical elements.
- Even the Lanthanides and Actinides, which are in the f-block, can be mentioned as transition metals. But because the f-orbitals of the f-block elements are not completely filled, they are often called Inner transition elements or metals. Below is a picture that shows where transition metals are on the periodic table and how their general electronic structures look.

- It’s important to remember that mercury, cadmium, and zinc are not considered transition elements because of how their electrons are arranged like (n-1)d10 ns2.
- In their ground states and even in some of their oxidation states, the d orbitals of these elements are all filled up. One example is the +2 oxidation state of mercury, which corresponds to an electronic configuration of (n-1)d10.
- Transition elements have certain points of resemblance. For example, they are all metallic in nature, play an important role in industries, are all hard and strong metals, form alloys, show variable oxidation states, and their compounds are mostly colored.
Electronic Configuration of Transition Element
Below is a table with a list of the first two rows of transition elements and the electronic configurations that go with them. It is important to note that the arrangement of electrons in some of these elements corresponds to (n-1)d5 ns1 or (n-1)d10 ns1. This is because the electron orbitals are stable because they are either half full or full.
| Transition Elements | Atomic Number | Electronic Configuration |
| Sc | 21 | [Ar] 3d1 4s2 |
| Ti | 22 | [Ar] 3d2 4s2 |
| V | 23 | [Ar] 3d3 4s2 |
| Cr | 24 | [Ar] 3d5 4s1 |
| Mn | 25 | [Ar] 3d5 4s2 |
| Fe | 26 | [Ar] 3d6 4s2 |
| Co | 27 | [Ar] 3d7 4s2 |
| Ni | 28 | [Ar] 3d8 4s2 |
| Cu | 29 | [Ar] 3d10 4s1 |
| Zn | 30 | [Ar] 3d10 4s2 |
| Y | 39 | [Kr] 4d1 5s2 |
| Zr | 40 | [Kr] 4d2 5s2 |
| Nb | 41 | [Kr] 4d4 5s1 |
| Mo | 42 | [Kr] 4d5 5s1 |
| Tc | 43 | [Kr] 4d5 5s2 |
| Ru | 44 | [Kr] 4d7 5s1 |
| Rh | 45 | [Kr] 4d8 5s1 |
| Pd | 46 | [Kr] 4d10 |
| Ag | 47 | [Kr] 4d10 5s1 |
| Cd | 48 | [Kr] 4d10 5s2 |
Complex compounds of Transition Element
- Transition elements make complex compounds by the attachment of a metal atom or ion with a certain electron donor species. Transition metal atom is mostly in the form of positive ion, whole negative or neutral ligands make the bonds with that to form a complex ion.
Examples: some common examples of Complex compounds of Transition Elements are [Ni(OH2)6]Cl2, hexaaquanickel(II) chloride (nickel cation before chloride anion), [Cu(NH3)6]Br2, hexaamminecopper(II) bromide (ammine ligand before copper metal), [Co(OH2)5Cl](NO3)2, pentaaquachlorocobalt(III) nitrate (aqua before chloro), etc.
- This complex ion may have a positive or a negative charge. It is written within the brackets and is called the coordination sphere. The number of ligands attached gives us the coordination number.
- Ligands can be monodentate, bidentate, tridentate, and even hexadentate. The charge on the coordination sphere depends on the charge on the central metal atom or ion and the nature of the ligand. If the ligand is bidentate or polydentate, then chelates are produced.
NOMENCLATURE OF COMPLEX COMPOUNDS
The nomenclature of complex compounds is based upon the recommendations of the Inorganic Nomenclature Committee of IUPAC.
Here are the rules for giving names to complex compounds:
- Cations are named before anions.
- In naming the coordination sphere, ligands are named in alphabetical order regardless of the nature and number of each, followed by the name of the central metal ion.
- The number of coordinated ligands is shown by a prefix such as di, tri, tetra, penta, hexa, etc.
- The names of anionic ligands end in the suffix O, e.g, hydroxo, (OH) carbonato (CO₂). Most of the names of neutral ligands don’t change. For example, NH is still called ammine, H2O is still called aqua, and CO is still called carbonyl.
- If the complex is an anion, the name of the metal is changed by adding “ate.” If it is not an anion, the name of the metal stays the same.
- A Roman number in parenthesis shows how many times the metal ion has been oxidized.
Properties of some common Transition Elements
Let us discuss Iron and Steel in this section
Iron
- The important ores of iron are used to get pure iron. The iron which is obtained is worked in three forms i.e. cast iron or pig iron, wrought, and steel. Cast iron has 2.52 4.5 % carbon, wrought iron has 0.12 – 0.25% carbon, and steel contains 0.25 -2.5 % carbon.
- Cast iron is converted to wrought iron, by using a puddling furnace. In this furnace, the iron oxide is reduced into iron metal and the impurity like carbon, sulfur, and silicon is oxidized.
- Manganese and phosphorous are converted into silicates and phosphates in the form of slag.
- Wrought iron is grey in color, extremely tough, resistant to corrosion, brittle, and can easily be welded. It can be worked under a hammer and can assume any desired shape.
Steel
- Steel has about the same amount of carbon as cast iron and more than wrought iron. It comes in three different kinds: low-carbon steel, medium steel, and high-carbon steel. These types depend on how much carbon they have and how they are used.
- Open hearth process or Bessemer’s process can manufacture steel. The heat economy of an open hearth furnace is based on a system of heat regeneration.
- The furnace can have an acidic lining of SiO₂ or a basic lining of dolomite.
- The impurities present in the pig iron and scrap steel which are oxidized in this furnace are carbon, silicon, manganese, sulfur, and phosphorous. In this way, SiO2, SO2, and P₂Os react with different types of metal oxides to give slag.
- Slag floats to the top of molten metals, where it is taken away. Finally, certain metals are added to get the steel of the required quality.
- In the Bessemer process, pig iron or cast iron is directly taken from the blast furnace and the blast of hot air is injected through the holes at the bottom.
- The substances like carbon, silicon, and manganese are converted to their oxides and finally to silicates. Carbon monoxide produced burns at the mouth of the converter with a blue flame. When the blue flame subsides, a mixture of carbon, iron, and manganese is added to get the steel of the required quality. In order to remove entrapped bubbles of gases, a little aluminum is added.
Why Transition Elements are less reactive?
Because they have a high melting point and a high ionization power, elements in the transition metal group are less likely to react.
Melting and Boiling Points of the Transition Element
The melting and boiling points of these things are high. This is because (n-1) “d” orbitals overlap and the electrons that don’t have a partner in a “d” orbital form covalent bonds. Zn, Cd, and Hg have all had (n-1) d orbitals that are completely full. They can’t make chemical bonds. So, their melting point is lower than that of other d-block elements. That’s why Transition Metals are less reactive.

Ionization power of the Transition Element
Ionization enthalpy is the amount of energy that an element needs in order to get rid of a valence electron. The element’s ionization potential goes up as the effective nuclear charge acting on the electrons goes up. Because of this, the enthalpies of ionization of transition elements are usually higher than those of s-block elements. That’s why Transition Metals are less reactive.
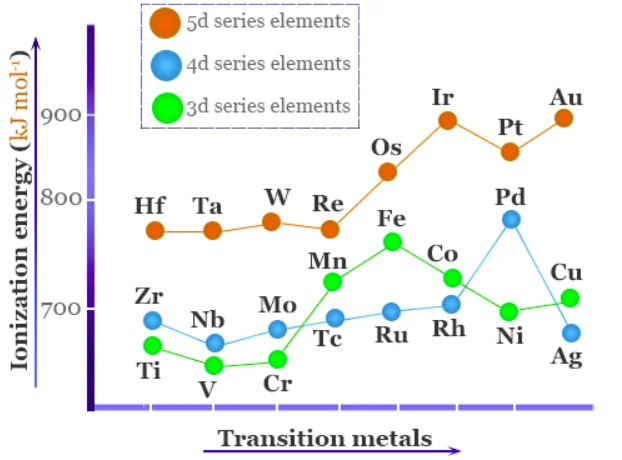
In a way, the atomic radius of an element is linked to its ionization energy. Ionization enthalpies tend to be higher for atoms with smaller radii than for atoms with relatively larger radii. As we move up and down the row, the ionization energies of the transition metals go up (due to the increase in atomic number). Corrosion is the best example of less reactivity of Transition Metals.
Corrosion
- During the process of corrosion, the surface of the metals is coated with oxides, sulphides, and carbonates.
- In the presence of water, the corrosion penetrates the metal. Two theories are there to explain the corrosion of metals i.e. acid theory and electrochemical theory.
- Coating of metals, alloying, metallic coating, phosphate coatings, and concrete coatings can prevent corrosion.
- Tin plating is also called cathode coating. If the protective coating of tin is damaged, iron comes in contact with the moisture and corrosion becomes more rapid. Galvanizing and zinc coating is also called anodé coating.
Advanced properties of Transition Elements
- The compounds and ions made from transition elements are colored. The d-d transition elements of electrons are what give this color its reason.
- The difference in energy between the different ways these transition elements can be oxidized is not very big. Because of this, the transition elements have many oxidation states.
- Due to the unpaired electrons in the d orbital, these transition elements combine to make many paramagnetic compounds.
- Many different kinds of ligands can bind to these elements. Because of this, transition elements are able to form a wide range of stable complexes.
- These elements have a lot of charge for how big they are.
- Most transition metals are hard and compared to other elements, they are pretty dense.
- Due to the involvement of the delocalized d electrons in metallic bonding, the melting and boiling points of these elements are high.
- The fact that the d electrons are spread out makes the transition elements good conductors of electricity.
- Atomic and ionic radii of transition elements decrease from left to the right in all the series of the d-block elements with a few abnormalities. The decrease in the values is not as prominent as for the representative elements due to lanthanide contraction.
- Their ionization potentials and electron affinities increase from left to right with a few exceptions. Their densities increase from left to right except for zinc, which shows abnormal behavior.
- Their melting and boiling points are closely associated with their binding energies. The melting and boiling points along with the binding energies increase from left to right up to the middle of the series and then they decrease and become minimum at the end of the series. This is all due to the varying number of unpaired electrons in their outermost orbitals.
- Transition elements are mostly paramagnetic in nature due to the presence of unpaired electrons. Unpaired electrons create spin magnetic movements. This property is greatly associated with the elements in the middle of the series due to the greater number of unpaired electrons.
- Transition elements show variable oxidation states due to the presence of
- unpaired electrons. This property increases up to the middle of the series and then decreases onward.
- The compounds of the transition elements are colored. The color is developed due to the transition of electrons between those d-orbitals, which have lost their degeneracy.
- Transition elements and their compounds are mostly used as catalysts. The catalytic properties are thought to be due to the unpaired electrons.
- The formation of the interstitial compounds is another important property of the transition elements. Small-sized atoms like hydrogen; oxygen, carbon, boron, etc. are accommodated in the interstices and give non-stoichiometric compounds.
- Similarly, transition elements give good alloys when different transition elements are mixed with each other in different ratios.
- Transition metals are always used as catalysts in different industries. When manganese and chromium, which are also transition metals, are added to iron, they make steel. Steel is used to make buildings, cars, planes, and other things. To make stainless steel, nickel is used.
- Transition metals and their compounds work as catalysts because they can change the oxidation state of other substances or, in the case of the metals, because they can attract other substances to their surface and make them work.
- Transition metals, like iron, copper, zinc, and manganese, are important parts of many biological processes in bacteria that help them adapt and evolve. As a way to invade a host, they are often involved in controlling how dangerous bacteria are.
- These alloys are very important to the world economy because they are used to make things like construction and building materials, tools, cars, cosmetics, paints, fertilizers, etc. Covers for manholes are often made out of cast iron.
FAQ’s
What is the Colour of d-block elements?
No color. In the visible region, there are no d-electrons and electrons cannot be excited. There is no color because all of the d-orbitals are full.
What is the first transition series of d-block elements?
The d-block elements are split into three groups. The first group is made up of the elements Sc through Cu. The second group is made up of the elements Y through Ag (the element La and the elements Hf through Au).
What is the full form of d-block elements?
On the modern periodic table, D block elements are found from the third group to the twelfth group. The d orbital is where the valence electrons of these elements can be found. You can also call the elements in the D block transition elements or transition metals.
Why all elements of d-block are metals?
Metals are also great because they are easy to mix. This is because the size of the atoms in all d-block metals is about the same. This makes it easy for them to switch places in a crystal lattice.
Where are the least reactive elements?
Group 18 of the periodic table is made up of noble gases. They don’t react with other things and aren’t metals. Noble gases are the ones that don’t react with anything. This is because their outer energy level is filled with eight valence electrons.
More Articles



1 Comment
What’s up, all the time i used to check webpage posts
here in the early hours in the break of day, as i enjoy to find out more and more.